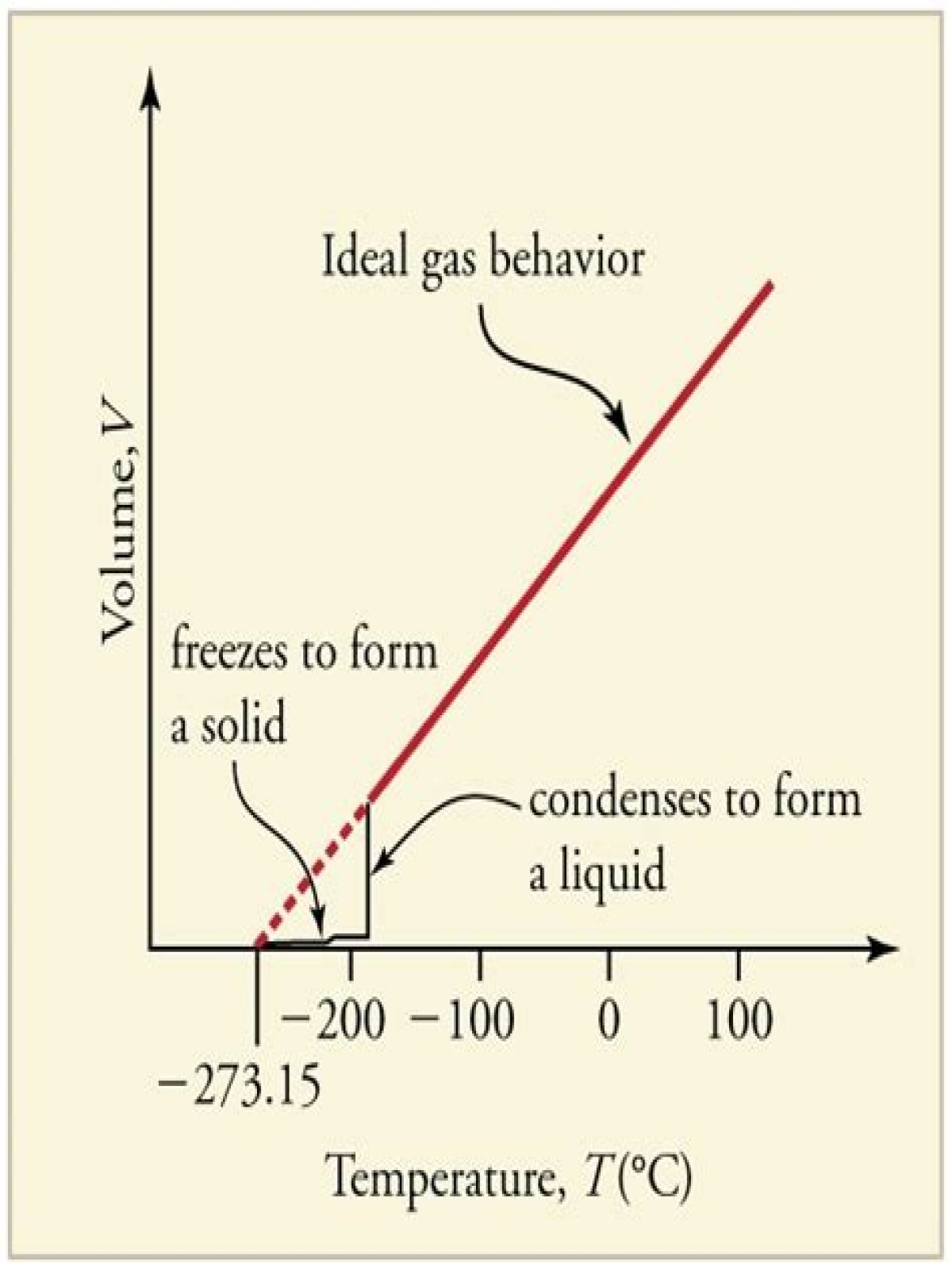
What happens to a substance’s temperature and a system’s energy during a phase change? The temperature of a substance does not change during a phase change. One way to recognize a phase change is by measuring the temperature of a substance as it is heated or cooled.
- Why does temp not increase during phase change?
- Are heat and temperature the same thing?
- What is the relationship between temperature heating or cooling and energy during a phase change?
- How is temperature related to the motion of molecules?
- What is the relationship between phase change and heat energy?
- Does the temperature change while either boiling or melting phase changes is occurring?
- What happens to the temperature of the substance while it is changing phase explain how that makes sense describe what is happening microscopically to the substance?
- What is the relationship between thermal energy and temperature?
- What is the similarities between heat and temperature?
- Is heat dependent on temperature?
- How does temperature cause changes in matter?
- What is the relationship of temperature to the collision of molecules?
- What affects temperature change?
- Does the temperature change at the melting and boiling points Why or why not?
- What happens when heat is added once the phase change is complete?
- Why does temperature remain constant during boiling?
- What is related to temperature?
- Why does the temperature remain the same during melting?
- How is temperature related to heat Quizizz?
- How are heat and temperature related What are the 3 major mechanisms of heat transfer?
- Are temperature and heat the same thing quizlet?
- How are heat and temperature not similar?
- What is the main role of temperature?
- How does temperature change when kinetic energy increases?
Why does temp not increase during phase change?
During a change of the state of matter, the supplied energy is not used to increase the kinetic energy of the molecules, but to change the binding energies. Therefore, the temperature remains constant.
Are heat and temperature the same thing?
In thermodynamics, heat and temperature are closely related concepts with precise definitions. Heat, qstart text, q, end text, is thermal energy transferred from a hotter system to a cooler system that are in contact. Temperature is a measure of the average kinetic energy of the atoms or molecules in the system.
What is the relationship between temperature heating or cooling and energy during a phase change?
Heat is the total energy contained within a substance. As a solid is heated, its temperature increases as the molecules move faster. During the phase change, when solid melts into liquid, its temperature remains constant as the heat energy is stored as potential energy.With an increase in temperature, the particles move faster as they gain kinetic energy, resulting in increased collision rates and an increased rate of diffusion. … With an increase in temperature, the particles gain kinetic energy and vibrate faster and more strongly.
What is the relationship between phase change and heat energy?
PHASE CHANGES Substances can change from one phase to another. When they do, energy (usually heat) is gained or lost. In this way, solids turn to liquids and liquids to gases when heat energy is gained or absorbed. When heat energy is lost (given off) gases change to liquids and liquids change to solids.
Does the temperature change while either boiling or melting phase changes is occurring?
To boil or melt one mole of a substance, a certain amount of energy is required. … But the heat added does not change the temperature; that heat energy is instead used to break intermolecular bonds and convert ice into water. At this point, there is a mixture of both ice and water.
What happens to the temperature of the substance while it is changing phase explain how that makes sense describe what is happening microscopically to the substance?
Temperature remains constant during phase change because at that point, any energy added to the substance is used to change its phase rather than to change the temperature. In other words, during phase change, the distance between the molecules changes, but not the translational velocity of such molecules or particles.What is the relationship between thermal energy and temperature?
Temperature measures the average kinetic energy of the particles in a substance. Thermal energy measures the total kinetic energy of the particles in a substance. The greater the motion of particles, the higher a substance’s temperature and thermal energy.
How are heat and temperature related quizlet?they are related because heat is the transfer of energy due to differences in temperature. Temperature is the measure of average kinetic energy of an atom or molecule in a substance. … the measure of average kinetic energy of an atom or molecule inside an object.
Article first time published onWhat is the similarities between heat and temperature?
Both heat and temperature are the concepts of thermodynamics; that works together to let the energy flow from hotter body to the cooler body. While heat depends on the number of particles in an object, temperature does not depend on a number of particles in an object because it is an average measurement.
Is heat dependent on temperature?
In general, the specific heat also depends on the temperature. The table below lists representative values of specific heat for various substances. Except for gases, the temperature and volume dependence of the specific heat of most substances is weak.
How does temperature cause changes in matter?
Physical conditions like temperature and pressure affect state of matter. … When thermal energy is added to a substance, its temperature increases, which can change its state from solid to liquid (melting), liquid to gas (vaporization), or solid to gas (sublimation).
What is the relationship of temperature to the collision of molecules?
As temperature increases, molecules gain energy and move faster and faster. Therefore, the greater the temperature, the higher the probability that molecules will be moving with the necessary activation energy for a reaction to occur upon collision.
What affects temperature change?
The factors that affects temperature are altitude, latitude and distance from sea. The height measured from sea level is called altitude. When the latitude increases, the distant from the sun also increases, so the temperature gradually decreases. When the altitude increases, the temperature also gradually decreases.
Does the temperature change at the melting and boiling points Why or why not?
The temperature of a substance remains constant at its melting and boiling points until all the substance melts or boils because, the heat supplied is continuously used up in changing the state of the substance by overcoming the forces of attraction between the particles.
What happens when heat is added once the phase change is complete?
As heat is added to solid water, the temperature increases until it reaches 0 °C, the melting point. At this point, the phase change, added heat goes into changing the state from a solid to liquid. Only when this phase change is complete, the temperature can increase.
Why does temperature remain constant during boiling?
During the boiling of water the temperature stays constant while heat is supplied continuously. It is because the heat provided by the water particles is consumed, and this heat increases their kinetic energy. … Therefore, the temperature stays constant only though heat is continually supplied to the water.
The temperature of a substance is closely related to the average kinetic energy of its molecules.
Why does the temperature remain the same during melting?
Answer: The ‘temperature of a substance’ remains constant during its melting and boiling point because the change in any state of matter as n solid to liquid or the liquid to a gas involves crossing the ‘latent heat of fusion’ which causes a change in the intermolecular spacing of the molecules in the substance.
How is temperature related to heat? Temperature is a measure of the heat of an object. Heat causes a change in the temperature of an object. Raising the temperature causes the heat of an object to increase.
List and describe the three major mechanisms of heat transfer in the atmosphere. Conduction: heat transfer through matter by particle activity. Convection: heat transfer by mass movement within a substance. Radiation: energy transfer by electromagnetic waves.
Are temperature and heat the same thing quizlet?
Are temperature and heat the same thing? No, temperature is the state of a system relating to how hot or cold it is, while heat is the transfer of thermal energy between two systems because they have different temperatures.
How are heat and temperature not similar?
Heat and temperature are two different quantities. The basic difference between heat and temperature is that Heat is the form of energy that transfers from a hot body to a cold body. Its unit is the joule. While the temperature is the degree of hotness and coldness of the body.
What is the main role of temperature?
Temperature. Temperature has the single most important influence on the distribution of organisms because it determines the physical state of water. Most organisms cannot live in conditions in which the temperature remains below 0 °C or above 45 °C for any length of time.
How does temperature change when kinetic energy increases?
When the average kinetic energy of its particles increases, the object’s thermal energy increases. Therefore, the thermal energy of an object increases as its temperature increases.